Active Learning Accelerates Electrolyte Discovery for Anode-Free Lithium Metal Batteries
Published on Quantum Server Networks
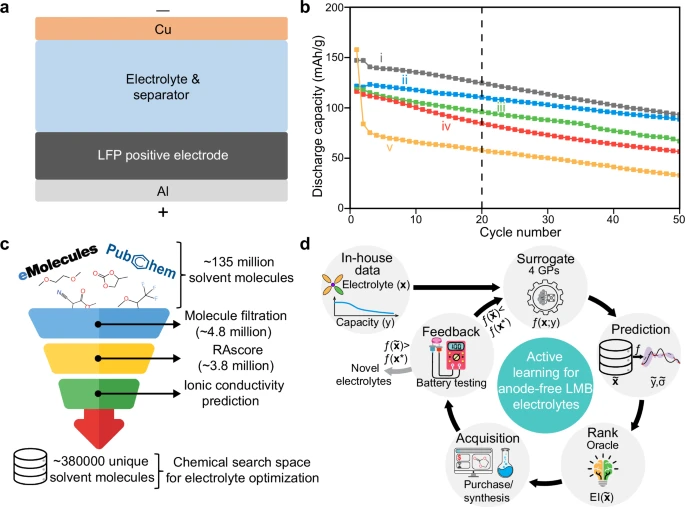
The pursuit of next-generation batteries capable of meeting the soaring demands of electric vehicles, aviation, and grid-scale energy storage has led researchers to explore anode-free lithium metal batteries (LMBs). These batteries promise significantly higher energy density than conventional lithium-ion systems by eliminating the bulky lithium metal anode and relying instead on lithium plating and stripping directly from a bare current collector. However, this architecture faces a critical bottleneck: cycle life. Without excess lithium, any inefficiency during plating and stripping quickly leads to capacity loss.
A groundbreaking new study published in Nature Communications introduces a transformative approach to this challenge. Researchers from the University of Chicago and collaborators demonstrate how active learning (AL) — a machine learning method rooted in Bayesian optimization — can accelerate the discovery of effective electrolytes for anode-free LMBs. (Read the original article here).
Why Electrolytes Matter in Anode-Free Designs
Electrolytes are central to battery performance because they directly influence the formation of the solid electrolyte interphase (SEI), a passivating layer that governs lithium metal stability. In anode-free LMBs, where there is no buffer of excess lithium, the SEI must enable near-perfect reversibility of deposition and stripping. To date, improvements have been incremental and largely trial-and-error, ranging from high-concentration ether-based formulations to fluorinated solvents that stabilize lithium cycling.
The Role of Active Learning
Unlike conventional machine learning, which requires large datasets, the active learning framework employed here thrives in data-scarce, noisy environments. Beginning with just 58 in-house electrolyte cycling profiles, the researchers trained a suite of Gaussian process models with Bayesian model averaging to balance predictive uncertainty. They then iteratively explored a massive search space of nearly one million electrolyte candidates sourced from chemical databases, narrowing down optimal solvents over multiple experimental rounds.
In just seven active learning campaigns, each testing about ten electrolytes, the algorithm homed in on a handful of promising solvents. Four stood out for their superior capacity retention in Cu||LiFePO4 cells, rivaling the best-known electrolyte systems in the field. Intriguingly, the framework displayed a consistent preference for ether-based solvents — a trend corroborated by both experimental validation and literature precedent.
From Virtual Space to Real Performance
The identified solvents not only matched state-of-the-art benchmarks in terms of capacity retention but also showed encouraging results in lithium deposition morphology and interfacial stability. Scanning electron microscopy revealed uniform, granular lithium plating, comparable to optimized fluorinated ether electrolytes. Additional analyses highlighted strong ion pairing and SEI compositions rich in inorganic species such as LiF and Li2O — hallmarks of stable lithium cycling.
These findings underscore the power of AI-assisted discovery: in a field where each experimental cycle is resource-intensive, active learning dramatically reduces the number of trials required to identify high-performing candidates.
Implications for Energy Storage
This work represents more than just an advance for anode-free lithium metal batteries. It signals the emergence of AI-driven materials design as a core tool for next-generation energy technologies. By efficiently navigating vast and complex chemical spaces, frameworks like this could be adapted to explore electrolytes for sodium-ion, magnesium, or even solid-state batteries. The integration of active learning with automated laboratories could usher in a new era of self-driving experiments, where discovery cycles are shortened from years to months.
Conclusion
Anode-free lithium metal batteries remain one of the most promising frontiers in energy storage. By leveraging active learning, researchers have taken a crucial step toward overcoming their key limitation: cycle life. The ability to screen vast electrolyte spaces with minimal data and experimental feedback may not only revolutionize how we discover battery chemistries but also reshape the landscape of computational materials science itself.
Original source: Nature Communications
*This blog article for Quantum Server Networks was prepared with the assistance of AI technologies.*
Sponsored by PWmat (Lonxun Quantum) – pioneers in GPU-accelerated materials simulation software supporting breakthroughs in quantum, energy, and semiconductor research. Discover more at: https://www.pwmat.com/
🚀 Explore our advanced simulation platform designed for high-performance computing in materials science.
🎁 Try PWmat today: Fill out our quick form to request a free trial and tailored R&D support: Request Free Trial
📞 Phone: +86 400-618-6006
📧 Email: support@pwmat.com
#ActiveLearning #LithiumMetalBatteries #ElectrolyteDiscovery #EnergyStorage #MaterialsScience #QuantumServerNetworks #BatteryResearch #MachineLearning #AIinScience #NextGenBatteries

Comments
Post a Comment