Hexanitrogen: The Most Energetic Molecule Ever Made—and It’s Stable at Liquid Nitrogen Temperatures
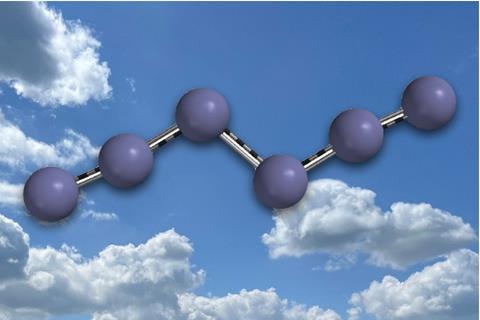
In a discovery hailed as “worthy of a Nobel prize,” chemists from Justus Liebig University Giessen in Germany have synthesized the first ever stable neutral nitrogen allotrope beyond dinitrogen. Known as hexanitrogen (N₆), this unprecedented molecule stores more energy than any previously known chemical compound and is stable—at least in cryogenic conditions.
Until now, nitrogen’s well-known stability came primarily from the strong triple bond in dinitrogen (N₂), the dominant form found in Earth’s atmosphere. Despite decades of speculation and theoretical work on polynitrogen species, no other stable neutral molecular nitrogen allotropes had ever been isolated. Now, that’s changed.
Redefining What’s Possible in Nitrogen Chemistry
The new N₆ molecule was synthesized by reacting chlorine gas with silver azide (AgN₃) under reduced pressure. This elegant sequence generated intermediate azides which then linked into a six-nitrogen chain structure. The most fragile part of the molecule—its so-called Achilles heel—is the bond connecting two N₃ groups. Yet, when rapidly cooled to liquid nitrogen temperatures, the molecule stabilizes with an estimated half-life of over 100 years.
“At room temperature, it exists for around 36 milliseconds—enough to isolate, trap, and cool it,” noted researcher Artur Mardyukov. His team, along with Peter Schreiner and Weiyu Qian, characterized the molecule using advanced electron density maps and calculated potential energy landscapes.
Unprecedented Energy Storage Potential
When hexanitrogen breaks down, it releases more than twice the energy per mass compared to HMX (octogen), the most powerful non-nuclear explosive known today. Even better, the only byproduct is harmless nitrogen gas (N₂), making N₆ a potential game-changer in propulsion and clean energy applications.
“This would be the most useful rocket fuel on the planet,” said Schreiner. “It doesn’t burn—it just explodes into a large volume of gas. And it’s non-corrosive, unlike hydrazine.”
Rewriting the Chemistry Textbooks
According to nitrogen pioneer Karl Christe, the discovery of N₆ represents a breakthrough on par with buckyballs and graphene in carbon chemistry—both Nobel-winning milestones. But unlike carbon allotropes, nitrogen forms have been far more elusive due to high energy barriers and instability.
Christe emphasized that N₆ has an energy difference of 185 kcal/mol relative to N₂, compared to only a few kcal/mol between graphite and other carbon allotropes. That means the challenge was not just synthetic—it was quantum mechanical.
What Comes Next?
Encouraged by this success, the research team now aims to attempt the synthesis of even larger nitrogen clusters, including a speculative decanitrogen (N₁₀). Such molecules would push the frontier of high-energy materials further and potentially unlock new regimes in propulsion and safe, compact energy storage.
🔗 Source: Chemistry World – Most energetic molecule ever made is stable in liquid nitrogen
#Hexanitrogen #N6Molecule #HighEnergyMaterials #RocketFuel #NitrogenChemistry #Polynitrogen #ChemicalExplosives #MaterialsScience #QuantumServerNetworks #CryogenicStability #EnergeticMaterials #NobelWorthy
Sponsored by PWmat (Lonxun Quantum) – a leading developer of GPU-accelerated materials simulation software for cutting-edge quantum, energy, and semiconductor research. Learn more about our solutions at: https://www.pwmat.com/
📞 Phone: +86 400-618-6006
📧 Email: support@pwmat.com

Comments
Post a Comment