Lighting the Way to Greener Plastics: Light-Activated Catalysis Breakthrough
In a powerful stride toward industrial sustainability, researchers at Northwestern University have developed a cutting-edge light-activated catalyst that could revolutionize the way we produce propylene—a vital precursor in the manufacture of polypropylene plastics.
This innovation holds promise to drastically reduce carbon emissions associated with the conventional production process. The traditional methods—like steam cracking—are incredibly energy-intensive and contribute significantly to global greenhouse gas emissions. In fact, the combined annual production of ethylene and propylene emits nearly 900 million tonnes of CO₂.
A New Light in Catalysis
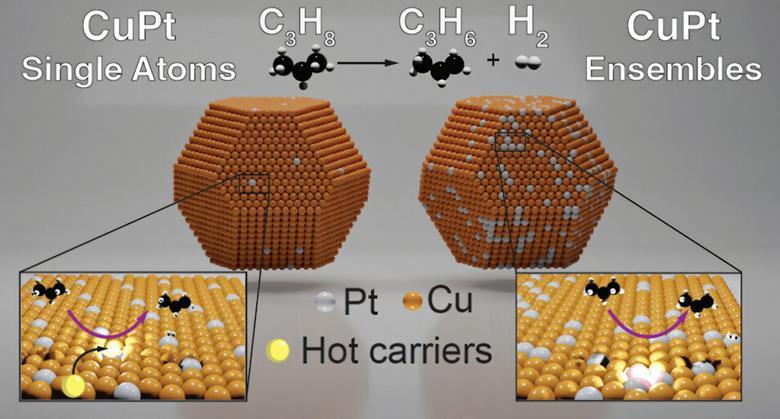
Published in the Journal of the American Chemical Society, the study showcases a photoactive alloy catalyst composed of copper doped with platinum. By harnessing visible light from LEDs or even sunlight, the catalyst efficiently performs non-oxidative propane dehydrogenation (PDH) — a cleaner route to producing propylene. This process operates under significantly milder conditions than current industry standards.
The research team discovered that atomically dispersed platinum sites served as highly reactive “hotspots” that effectively break C-H bonds, a critical step in converting propane to propylene. According to lead author Emma-Rose Newmeyer, these single-atom platinum sites enabled reactions at temperatures 50°C lower than traditional thermal methods.
Why This Matters
Propylene is essential for making countless products—packaging, fibers, automotive parts—and with over 150 million tonnes produced annually, a greener synthesis process could make a significant environmental impact. Using light instead of fossil-fuel-based heat could transform chemical manufacturing as we know it.
Senior author Dayne Swearer emphasized, “This study was really trying to leverage the idea of using light to power chemical processes.” This new understanding of how light can change chemical reaction pathways may even pave the way for future breakthroughs—like the direct conversion of methane to hydrogen and propylene.
What’s Next?
The team is currently exploring how the same principle could be applied to other alkanes, particularly more stubborn molecules like methane. While promising, some challenges remain, such as the catalyst's longevity. According to Jimmy Faria Albanese of the University of Twente, the activity of the photocatalyst currently degrades over time, which could affect its scalability for large industrial applications.
Still, the findings represent a critical step toward carbon-neutral industrial chemistry—proof that we may soon harness the power of light to manufacture essential chemicals with minimal environmental cost.
🔗 Read the full article: Improving propylene synthesis using light-activated catalysis could cut emissions (Chemistry World)
🚀 Sponsored Announcement
Introducing the Daily Hub for Materials Science Innovation!
🔬 Visit: https://quantum-server-materials.blogspot.com/
I’m excited to launch a new daily blog featuring the latest breakthroughs in materials science—from quantum materials to real-world energy solutions. Perfect for researchers, innovators, and industry leaders.
📢 Sponsorship Opportunities Now Open! Promote your:
- Software, R&D services, and materials technologies
- Research publications and laboratory innovations
- Events, workshops, or career opportunities
✅ Reach 200,000+ professionals through daily posts shared across LinkedIn and Facebook’s top scientific communities.
📲 Learn more: https://www.qscomputing.com
💬 Contact: gabriele.mogni@qscomputing.com
#MaterialsScience #Innovation #R&D #QuantumMaterials #SponsoredContent

Comments
Post a Comment